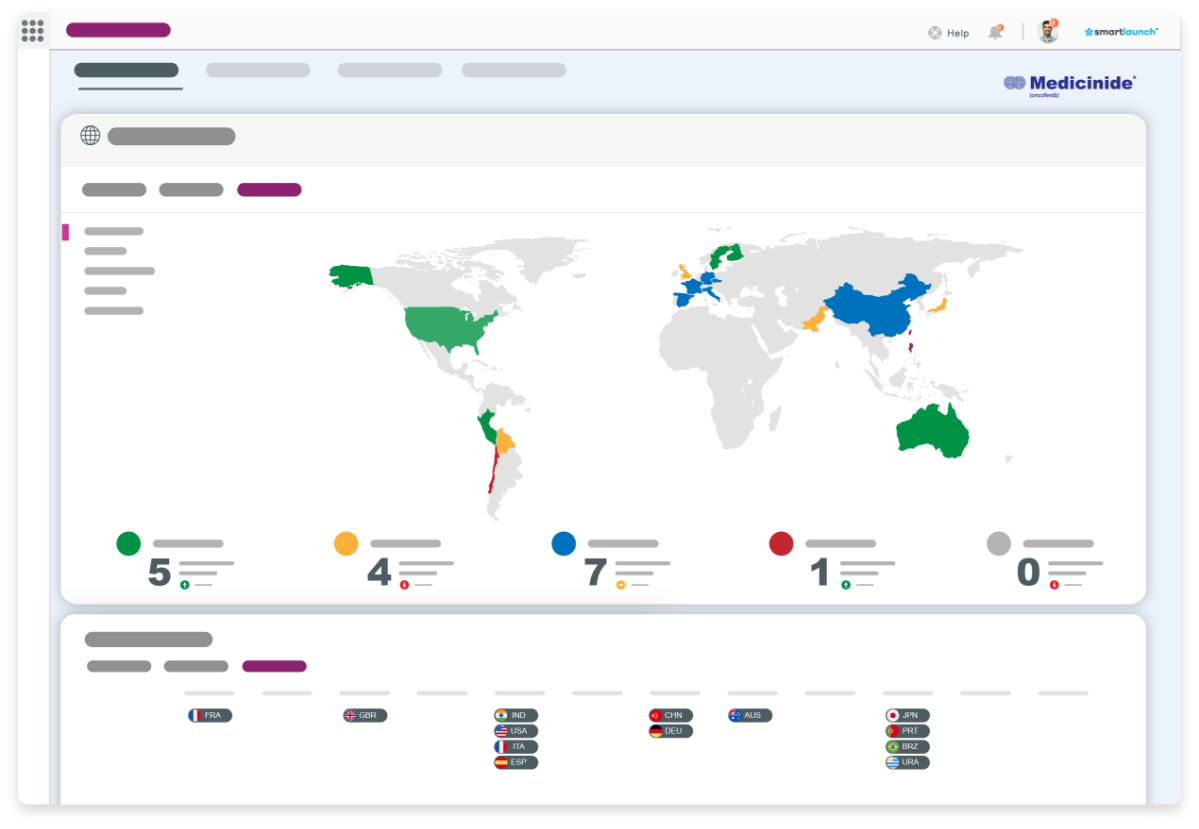
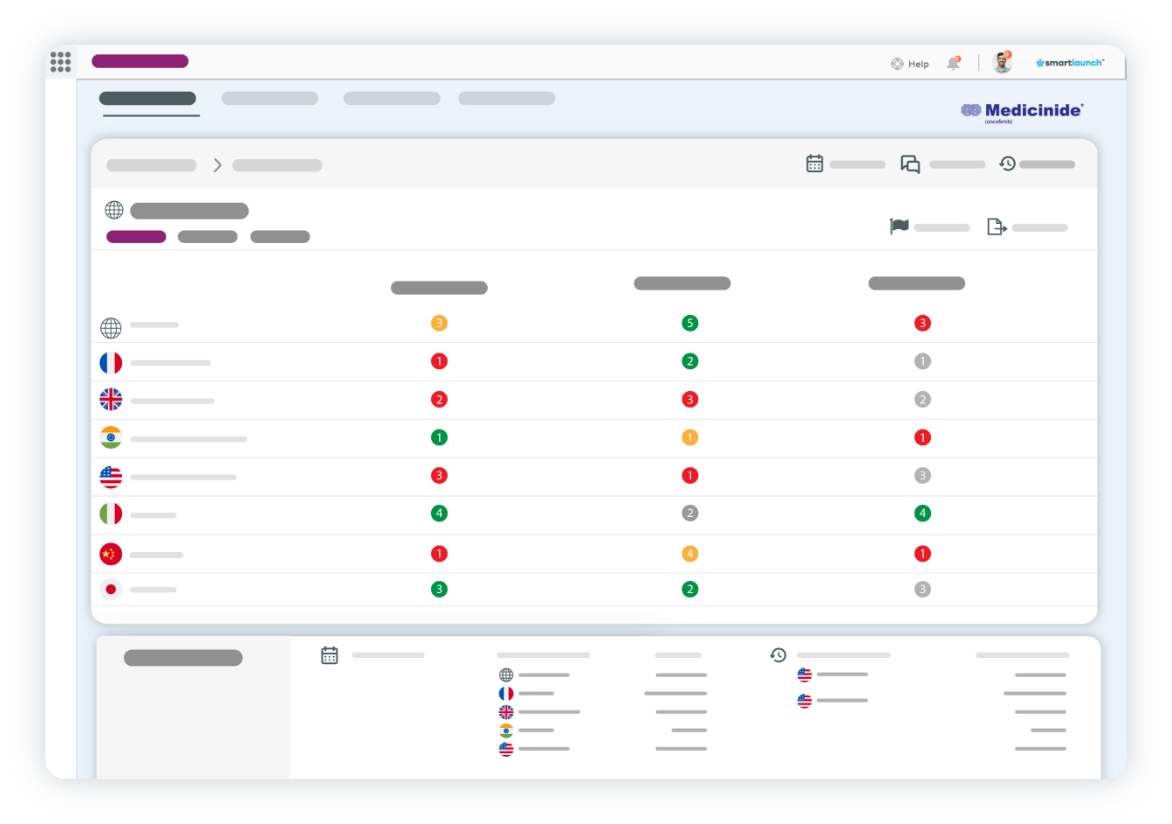
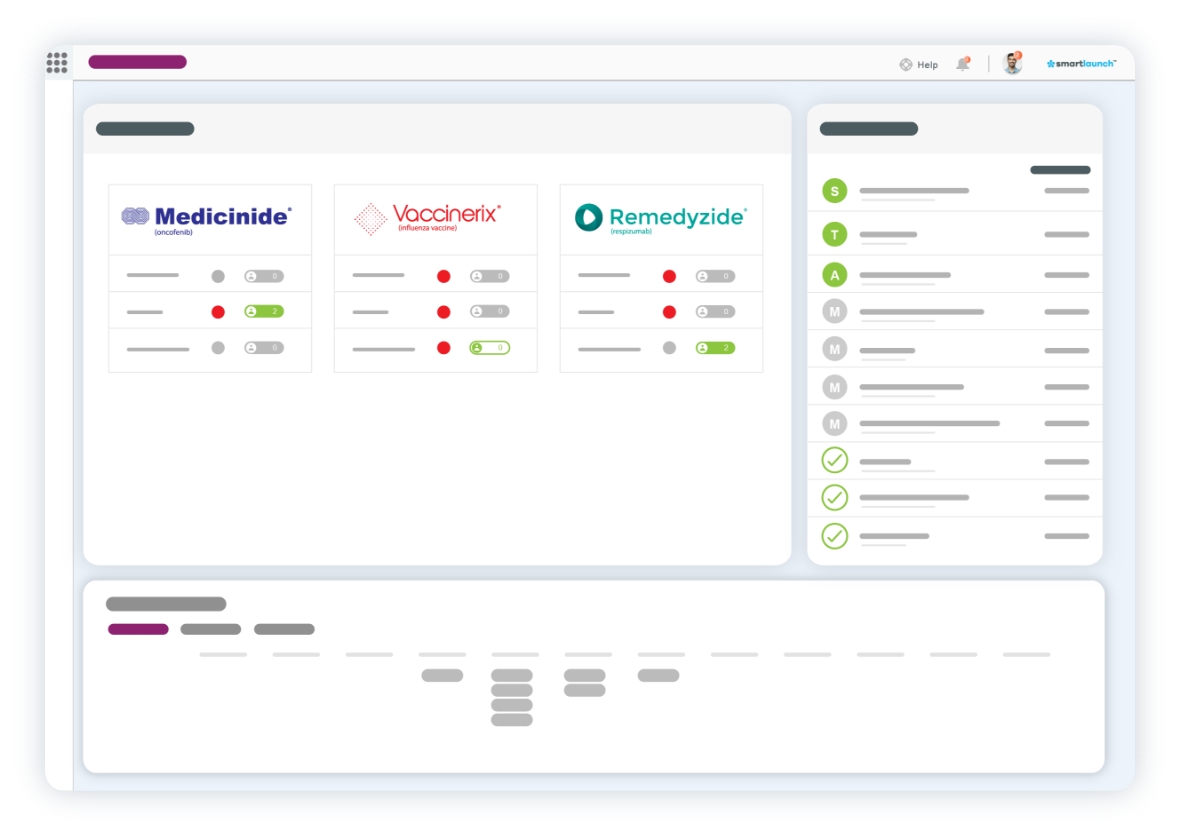
You have an exciting new pharmaceutical product poised to hit an expectant market after years of effort. Your launch readiness strategy is firing on all cylinders – or is it? If that question leaves you in any doubt, we can help put your mind at rest.
At TRiBECA® Knowledge, we deliver pioneering launch readiness software that helps pharmaceutical and biotech companies introduce new products to the global marketplace both faster and more efficiently.
A new product launch in pharmaceuticals is the critical moment when all of your hard work in research and development, regulatory affairs, pricing and reimbursement, health economics, marketing, advertising, promotion and media relations comes to a head. At that point, you want to be delivering the launch plan template at the top of your game.
So optimising launch readiness, and multiplying your chances of turning an also-ran into a new product launch that really justifies the investment, should be a no-brainer. Yet so many new product introductions, for whatever reasons, still fall short of expectations – if they get to launch at all. The rate of attrition in pharmaceutical research and development is notoriously high. Fewer than one in 10 candidate drugs ever gain marketing approval.
When a pharmaceutical product does make it through the long haul of drug discovery, compound selection, preclinical/clinical trials and regulatory sign-offs, it is absolutely imperative that launches across multiple markets are handled as quickly, seamlessly, efficiently and cost-effectively as possible. Launch excellence must be the binding principle in every activity contributing to that goal.































 André Moa
André Moa
 4 April 2022
4 April 2022
 9 minute read
9 minute read