- Our Products
-
 An agile launch execution platform, with full visibility across countries and
functions.
An agile launch execution platform, with full visibility across countries and
functions.
-
 A collaborative platform for teams who plan reimbursements and HTA activities.
A collaborative platform for teams who plan reimbursements and HTA activities.
-
 A collaborative platform designed to manage pharmaceutical tenders across
countries.
A collaborative platform designed to manage pharmaceutical tenders across
countries.
-
 A capability management platform that unifies capabilities, resources, and assessment.
A capability management platform that unifies capabilities, resources, and assessment.
-
 Manage your entire pipeline. Track every move. Stay ahead, from discovery to launch.
Manage your entire pipeline. Track every move. Stay ahead, from discovery to launch.
-
- Our Expertise
- Free Guides
- Blog
- Contact
Our products
Launch Readiness 2025

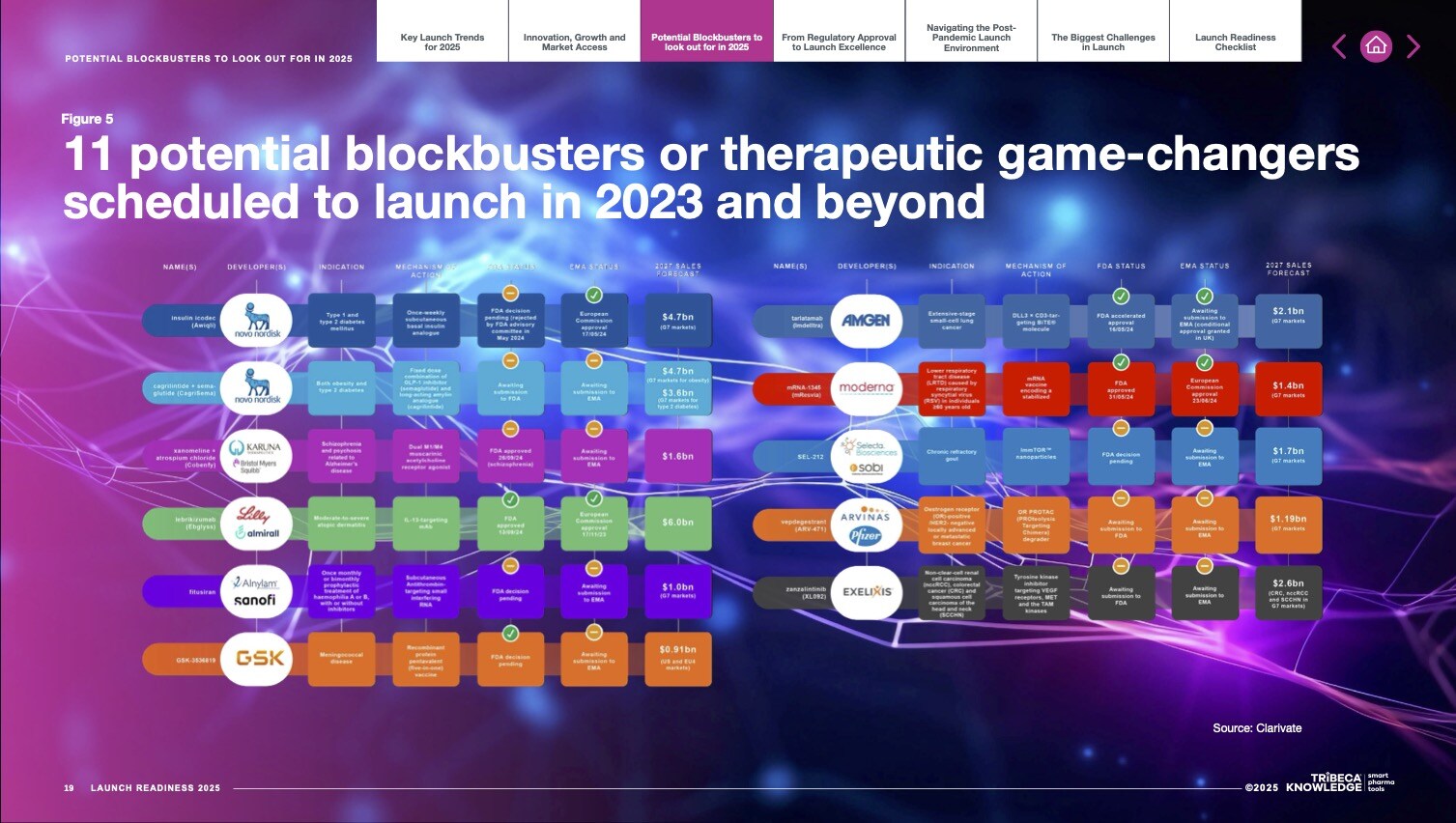



Launching a New Medicine in 2025? This Report is Essential.
The Launch Readiness 2025 report is your definitive guide to navigating the evolving pharmaceutical launch landscape. Packed with data-driven insights and actionable strategies, this must-read resource covers:
- The most anticipated new product launches in 2025.
- Recent key FDA and EMA approvals and what they mean for your pipeline.
- Major market access challenges—from pricing pressures to evolving HTA frameworks—and how to tackle them.
- Post-pandemic launch trends and the impact of digital transformation on commercial success.
- The Ultimate Launch Readiness Checklist—assess your organisation’s preparedness for a seamless, efficient, and successful product launch.
Make informed, confident decisions with expert insights from TRiBECA® Knowledge.
Download your copy today.
Enter your details below to download the report

