
A healthy pharmaceutical industry runs on innovation. There is plenty of that left in the tank, judging by current market trends and product development pipelines. Pharma launch challenges, though, have never been tougher.
Innovations in genomics, biology or diagnostics have given us safer, more effective medicines for many previously intractable or poorly served conditions. New speciality drugs for cancer, diabetes, multiple sclerosis, hepatitis C and a host of rare diseases are coming thick and fast.
Nine of the 22 novel pharmaceutical products approved by the US Food and Drug Administration in 2016 were orphan drugs. Yet this product strategy carries a heavy price for struggling healthcare systems.
For the top 10 medicines in the US last year, the average annual cost per patient for an orphan drug was $140,443 compared with $27,756 for a non-orphan product, notes a report by Evaluate Pharma.
In response, payers and health-technology assessors are taking an increasingly hard line on pricing, reimbursement and treatment access, informed by health economics and determined to get value they can afford.
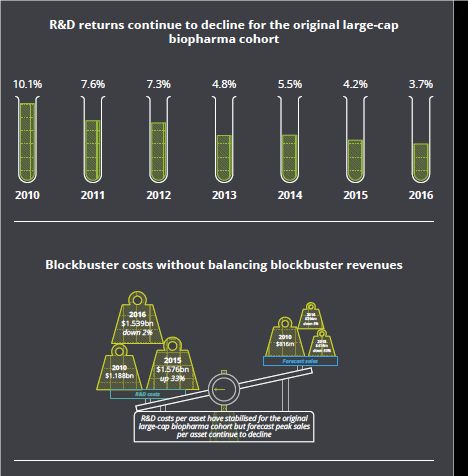
This is cutting into margins. The latest annual report from Deloitte found returns from pharmaceutical R&D investment at 12 leading biopharma companies were down to 3.7% in 2016, compared with 5.5% in 2014 and 4.2% in 2015. Peak sales per asset have fallen by 11.4% year-on-year since 2010.
 Source: Deloitte
Source: Deloitte
It is not just payers holding the reins. Pharmaceutical marketing must cover a plethora of stakeholders, teasing out the benefits of complex products through multiple channels in an enormously diverse global marketplace.
All of this demands new skills from teams under pressure to deliver more cost-effectively. Broad-brush platforms with armies of sales reps are out; high-level dialogue and key account management are in.
Increasingly, launch teams must do the same or better with less. Smaller-scale launches do not necessarily mean less effort. And with precision medicines relying on clusters of successive new indications, the pace can be all the more relentless.
This may be one reason why approval tallies are faltering. The 22 novel drugs cleared by the FDA in 2016 marked the agency’s lowest approval score since 2010; in 2015 it OK’d 45 novel medicines. The European Medicines Agency recommended just 27 new active substances for approval against 39 in 2015, its lowest ebb since 2011.
So is pharma taking a step back? With approval cycles subject to numerous variables, drawing definitive conclusions from these figures would be unwise. Coupled with Deloitte’s findings, though, they present a stark picture: fewer drugs are getting out there; when they do, the payback – at least for large multinationals – is less and less attractive.
Innovation in medicines is both desirable and commendable, but it is rarely enough in itself. For those products that make it, companies must work harder than ever to ensure product launch steps are optimally communicated, aligned and streamlined at every level of the organisation and in every market they enter.
TRiBECA® Knowledge is a market leader in smart business tools that help pharmaceutical companies optimise launch readiness by enhancing visibility, transparency, communication and collaboration across brands, management layers, business functions and countries worldwide.







 Andre Moa
Andre Moa
 27 Apr 2017
27 Apr 2017
 4 minute read
4 minute read