
Access to medicines is a loaded term. In an ideal world, every patient would have unimpeded access to the best drug available. In reality, access is usually selective at best.
We live with imperfect systems in which tough decisions must be made about comparative drug value and equitable distribution of healthcare resources.
In this context, pharmaceutical companies and other stakeholders, including patient groups, regularly invoke access to medicines as a measure of regulatory and economic barriers between medicines and end-users.
For example, the Association of the British Pharmaceutical Industry recently ran a snap poll of members’ reactions to new budget-impact thresholds introduced in the NHS for medicines deemed cost-effective by the National Institute for Health & Care Excellence.
It found that 71% of respondents believed their companies would now prioritise launching new medicines in European countries over the UK. Moreover, 89% thought the budget ceilings would reduce patient access to cost-effective medicines in the UK.
Market access barriers typically cited by industry include pricing and reimbursement procedures, health-technology assessments and negotiations with payers (see Figure 1).
Figure 1: Average time (in months) from EMA regulatory approval to first sales, and from first sales to post-marketing authorisation (PMA), in EU5 markets, 2015
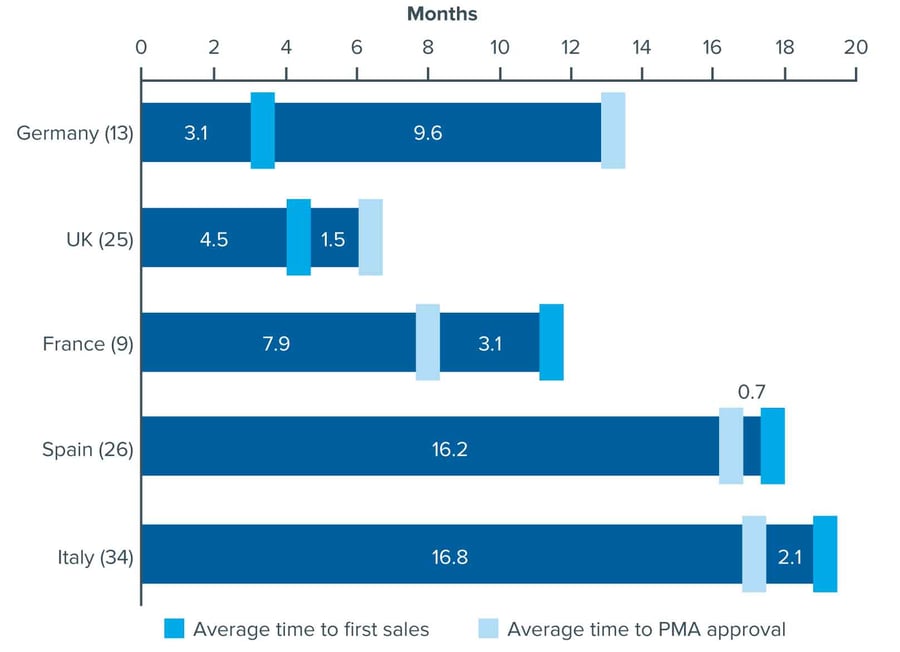
Source: QuintilesIMS Consulting Service analysis
Non-profit organisations, such as the Netherlands-based Access to Medicine Foundation, see things differently, though.
The Foundation monitors and indexes companies’ efforts to expand access to medicines in low- and middle-income countries. One criterion it uses is pricing. Indeed, industry critics regard unsustainable prices as a key variable affecting equitable patient access to medicines. Nor are patent holders solely to blame.
While at best they work for the greater good, drug companies are also competitive businesses whose influence on access to medicines is inevitably selective as well.
They may choose not to market a product where pricing and reimbursement are unfavourable. Product development may be oriented to conditions affecting people who can afford to treat them. And faced with a cheaper generic or biosimilar, research-based companies will often do everything in their power to delay market entry.
Complex equation
Ultimately, access to medicines is a complex equation involving multiple actors, each with their own agenda. Even patient organisations vie for limited funds with other groups focused on different diseases.
Yet the principle holds: the only truly effective drug is one that reaches the right patient at the right time under the right conditions. Without timely access to new therapeutic options, patients have fewer opportunities to manage disease, improve quality of life, extend life, and take the strain off family, friends and carers.
A recent French study looked at the relationship between novel cancer therapies and mortality in 11 developed countries. The researchers found that the availability of one new treatment for a particular cancer site (e.g., lung, breast) was associated with an average decline of 8-9% in mortality.
According to research presented at the 16th World Conference on Lung Cancer in 2015, every hour lost to the regulatory process for cancer drugs costs 29 life-years in North America and 260 worldwide.
Swiftly and seamlessly
This is one more reason for pharmaceutical companies to ensure they have a launch readiness framework fit for purpose in an environment where market access challenges come from many quarters, both external and internal.
If they want to be serious about patient access, and a new era of patient-oriented medicine, companies must do everything possible to facilitate launches that flow swiftly and seamlessly from one market to the next.
TRiBECA® Knowledge is a market leader in smart business tools that help pharmaceutical companies successfully launch and commercialise products. Our tools enhance visibility and transparency, streamline processes, utilise business intelligence better, and drive communication and collaboration across brands, management layers, business functions and countries worldwide.







 Andre Moa
Andre Moa
 25 May 2017
25 May 2017
 4 minute read
4 minute read