
Efficient and effective pharma launch planning is all about making sure your new product hits target markets with maximum impact and minimal complications – all in good time and at an acceptable cost.
A fully integrated launch plan, coupled with technology that enables cross-functional and pan-national communication and collaboration, will keep internal stakeholders on the same page while fostering global-local alignment throughout the product roll-out.
As consultants QuintilesIMS noted in a recent white paper, “the most fundamental foundational success factor remains alignment of the organisation behind the launch”.
True alignment, the paper adds, not only spans objectives, functions and countries. It also means “agreement on strategic objectives and critical success factors for the product […] and the Key Performance Indicators to measure these”.
Indeed, the strategic drivers behind a successful launch must constantly be re-assessed in the face of evolving market access challenges as a drug progresses from one country to the next.
An astute product launch plan will put that strategy into practice as effectively and consistently as possible. But the launch strategy itself has to be robust, detailed and agile enough to withstand the escalating complexities of today’s pharmaceutical market (see Figure 1).
In a modern pharmaceutical operating model, business strategy “must be explicitly spelled out and coherent across the critical teams, and aligned closely with business objectives that are regularly validated against shifting requirements and regulations in the industry”, point out Jo Pisani, and Dr. Myrto Lee of Strategy&, PwC’s strategy consulting group.
Figure 1: The rapidly changing environment for pharmaceutical launches
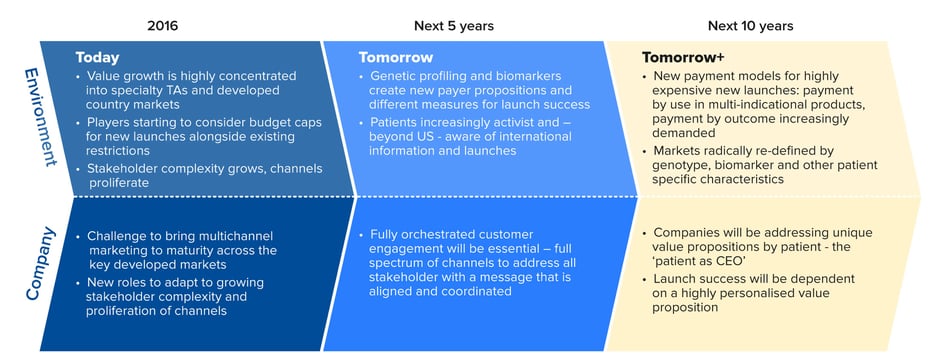
Source: Quintiles/IMS
Administrative distractions
What happens too often is that key personnel are caught up in time-consuming administrative distractions, such as collecting, processing, formatting and consolidating the data that underpin the launch plan.
The effects are aggravated when companies are managing and tracking their launch activities in Excel and Microsoft Project.
These tools are cumbersome and time-consuming to use, without the real-time data, nor the visibility across functions and countries, that streamline and optimise launch efforts. Lack of an audit trail, and less stringent controls on security and access, create further complications where tools should be facilitating the launch process.
Critical differentiation
When TRiBECA® Knowledge recently asked 450 pharma executives about their biggest challenges in launch planning, a number complained of inadequate resources and problems with resource allocation, budgeting and alignment, as well as difficulties with prioritising launch activities, strategic planning and time management.
Informed strategic input also provides critical differentiation in the marketplace. Despite the recent surge in speciality and orphan-drug launches, product differentiation remains an issue as R&D efforts cluster around the same potentially lucrative disease targets and unmet needs.
“What happened to the primary care market is now happening in specialty,” observes QuintilesIMS. “The maturation of many specialty therapy areas […] has started, and will accelerate over the next decade. This means the variety of situations which new specialty launches will encounter will increase, with many more having less differentiation and launching into areas with lower unmet need.”
Seamless flow
With the right launch readiness technology in place, data, information and insights can flow seamlessly across functional and national boundaries in real-time throughout your organisation to aid decision making. In that way, companies can ensure that the various human and intellectual resources on your launch team are deployed to optimal effect for a successful launch.
Importantly, team strategists can get on with what they do best: determining how new drugs can address the variable needs of patients, healthcare professionals and payers, rather than struggling to manually collect and reconcile data, which can easily become a distraction from executing the launch strategy with excellence.
TRiBECA® Knowledge is a market leader in smart business tools that help pharmaceutical companies successfully launch and commercialise products. Our tools enhance visibility and transparency, streamline processes and drive communication and collaboration across brands, management layers, business functions and countries worldwide.






 Andre Moa
Andre Moa
 22 Jun 2017
22 Jun 2017
 5 minute read
5 minute read